Our Research Mission
Imaging and biomedical engineering have enabled major breakthroughs in respiratory medicine. However, the causes for the emergence of heterogeneity in lung disease and injury, its progression, and responses to treatments remain largely unknown. At the Pulmonary Imaging and Bioengineering Laboratories, founded by Dr. Jose Venegas, we focus on understanding fundamental mechanisms in lung diseases and injury and their relationship to topographical heterogeneity. The ultimate goal of our research studies is to develop novel treatments and improve patient outcomes.
Pulmonary Imaging
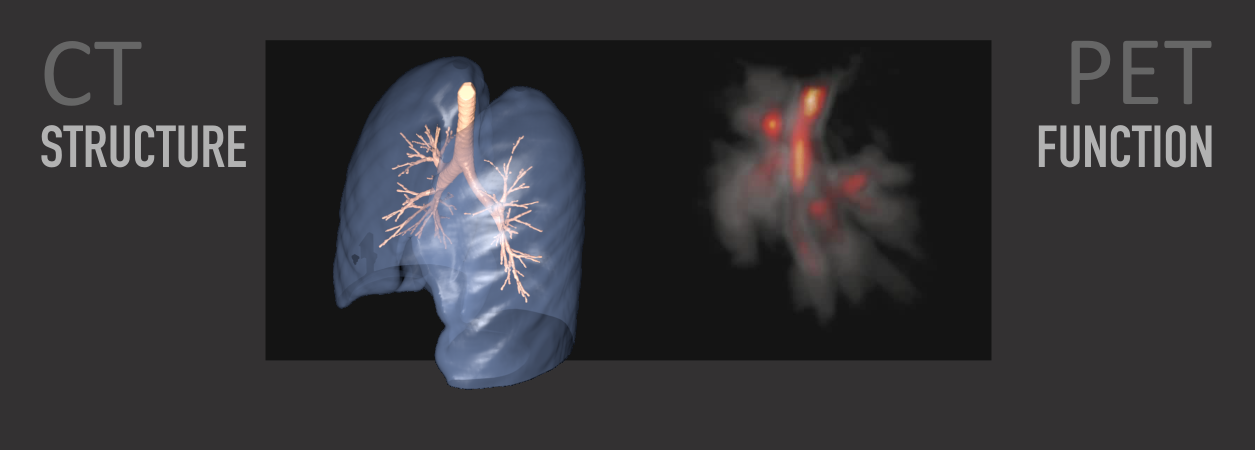
Our interdisciplinary team of physicians and engineers has developed advanced imaging methods and modalities that allow us to study a wide range of respiratory diseases and conditions in humans and large animals. Combining Positron Emission Tomography (PET) and Computed Tomography (CT) allows for both structural assessments using the high spatial resolution of CT and functional assessments using the excellent sensitivity of PET scanners for tracers and their kinetics.
Examples of innovations in imaging from our group include a highly accurate and unique technique of ventilation and perfusion imaging using the 13N-Nitrogen bolus-injection method pioneered by Dr. Venegas. We have also developed a patented method for calibrated image-derived input functions relevant for 18F-FDG kinetics, and a novel method for the assessment of the extravascular-extracellular volume in the lungs under conditions of regional edema and alveolar flooding.
Using quantitative PET-CT imaging, we have studied a substantial range of lung diseases and conditions including ARDS, asthma, COPD, healthy subjects, HIV, pulmonary arterial hypertension, pulmonary embolism, smoking, and ventilator-induced lung injury. The assessments in these studies included ventilation, perfusion, strain, 18F-FDG uptake linked to pulmonary inflammation, tissue volume, gas volume, gas fraction, aerosol deposition, blood volume, blood clotting, and other parameters characterizing disease-specific deviations from healthy conditions.
Bioengineering
The close collaboration between engineers and physicians is essential for the success of our projects allowing us to identify the biologically and medically relevant processes and apply engineering methods in our investigations. Our most successful modeling project was a computational model of bronchoconstriction in an airway tree that could explain our findings of ventilation defects (VDefs) in PET images and showed for the first time that there is a tipping point beyond which asthma attacks lead to avalanche-like constrictions and self-organized VDefs.
We have developed PET tracer models and parameter estimation methods as key elements of our quantitative PET-CT image analysis and built an extensive toolbox of custom software (Matlab) with integrated bridges to the available open-source software 3D Slicer and ANTs. Last but not least, we build custom devices for our projects.
Latest News
Welcome to our new website
We are very excited about the launch of our new website and the new opportunities for sharing our innovative projects. Our group has a long history of very successful research projects being a testimony of advanced interdisciplinary collaborations between physicians and engineers in our research center and a dynamic team with many brilliant fellows, post-docs, Ph.D. students, and undergraduates.
New Investigator Profiles at the Department of Anesthesia, Critical Care and Pain Medicine
Investigator Profiles is a new hub of all investigators and their profiles with an integrated search tool. It incorporates for the first time the personal profile pages of the 90+ investigators with faculty positions in our department's website Research in the Department of Anesthesia, Critical Care and Pain Medicine. This is a major new presentation of the extensive spectrum of the research in our department, and we proudly contributed to the development.
Presentions at the 2025 International Workshop on Pulmonary Imaging

PIBL investigators Dr. Tilo Winkler, Dr. Maurizio Cereda, Dr. Marcos F. Vidal Melo, and Dr. Yi Xin are invited to give talks at the 2025 International Workshop on Pulmonary Imaging in Philadelphia, PA. Check the meeting's program for details.
New Publications
Research | Anesthesiology. 2025 Feb 12. doi: 10.1097/ALN.0000000000005412 Online ahead of print. PMID: 39946655
Effects of lung expansion on global and regional pulmonary blood volume in a sheep model of acute lung injury
Mingyang Zang, Congli Zeng, David Lagier, Nan Leng, Kira Grogg, Gabriel Motta-Ribeiro, Andrew F Laine, Tilo Winkler, Marcos F Vidal Melo.
CONCLUSIONS: During low-volume mechanical ventilation and systemic endotoxemia, lung blood volume is markedly heterogeneously distributed, and modulated by PEEP. Nondependent regions are susceptible to low blood volume and capillary closure. Recruitment of pulmonary vascular blood volume with gas volume is nonlinear, limited at intermediate PEEP indicating its advantage to spatial distribution of blood volume.
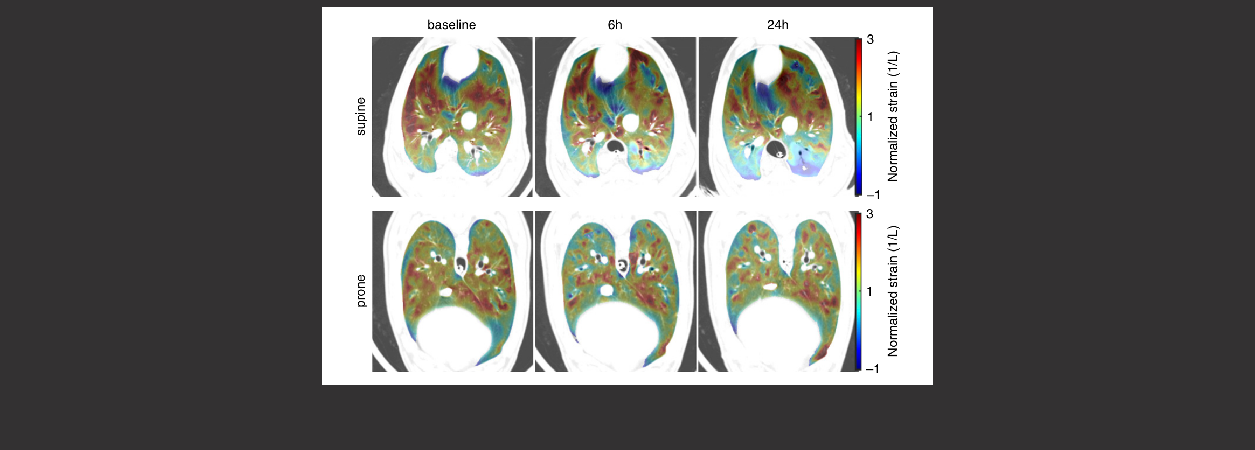
Research | Sci Transl Med. (2024)16(760):eado1097. doi: 10.1126/scitranslmed.ado1097 PMID: 39141699
Mechanical ventilation guided by driving pressure optimizes local pulmonary biomechanics in an ovine model
David Lagier, Congli Zeng, David W Kaczka, Min Zhu, Kira Grogg, Sarah E Gerard, Joseph M Reinhardt, Gabriel C Motta Ribeiro, Azman Rashid, Tilo Winkler, Marcos F Vidal Melo.
CONCLUSIONS: These findings advance the understanding of lung collapse, tidal overdistension, and strain heterogeneity as local triggers of ventilator-induced lung injury in large-animal lungs similar to those of humans and could inform the clinical management of mechanical ventilation to improve local lung biomechanics.
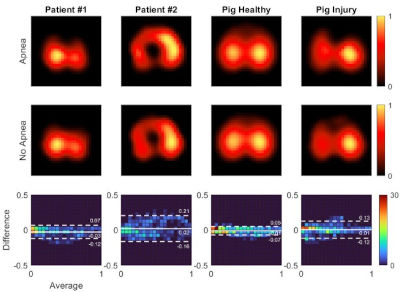
Research | Am J Respir Crit Care Med. (2024)209:1263-1265. doi: 10.1164/rccm.202310-1919LE PMID: 38412326
First-Pass Kinetics Model to Estimate Pulmonary Perfusion by Electrical Impedance Tomography During Uninterrupted Breathing
Marcus Victor, Yi Xin, Glasiele Alcala, Timothy Gaulton, Eduardo Costa, Tilo Winkler, Lorenzo Berra, Marcelo Amato, Maurizio Cereda.
CONCLUSIONS: We aimed to address a primary challenge in estimating regional pulmonary perfusion with EIT during controlled ventilation. Our findings demonstrate that EIT signal decomposition of saline injections during steady ventilation yields assessments of regional perfusion comparable to EIT perfusion during apnea in a cohort of patients and healthy and injured pigs.
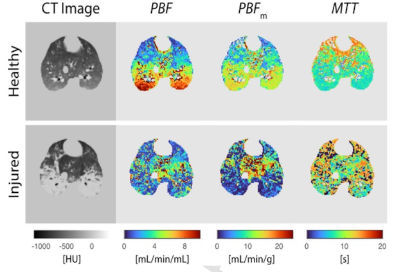
Review | Nitric Oxide. (2024)147:6-12. doi: 10.1016/j.niox.2024.04.004 PMID: 38588918
Imaging the pulmonary vasculature in acute respiratory distress syndrome
Timothy G Gaulton, Yi Xin, Marcus Victor, Alice Nova, Maurizio Cereda.
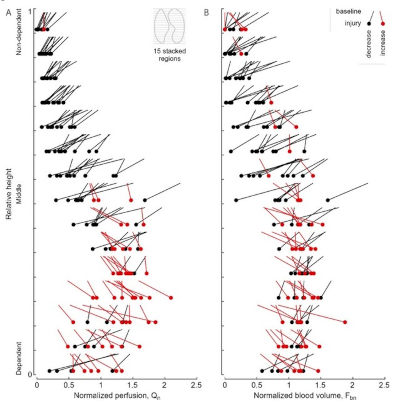
Research | Sci Rep. (2024)14:5832. doi: 10.1038/s41598-024-56565-6 PMID: 38461172
Regional pulmonary perfusion, blood volume, and their relationship change in experimental early ARDS
Arnoldo Santos, Gabriel C Motta-Ribeiro, Nicolas de Prost, Mauro R Tucci, Tyler J Wellman, Marcos F Vidal Melo, Tilo Winkler.
CONCLUSIONS: Endotoxine-associated early ARDS changed the relationship between blood volume and perfusion, shifting from linear to curvilinear. Effects of endotoxin exposure on the vasoactive blood flow regulation were most likely the key factor for this change limiting the quantitative accuracy of blood volume imaging as a surrogate for regional perfusion.
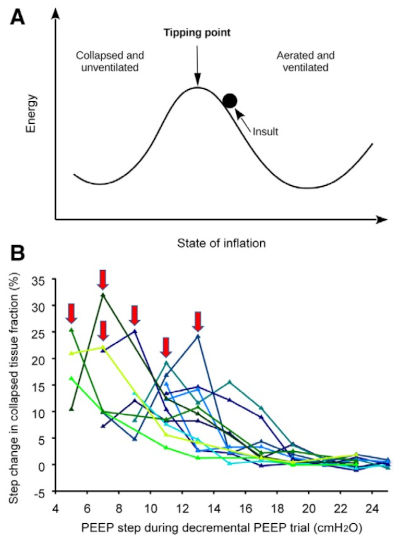
Editorial | Anesthesiology. (2023)139:719-721. doi: 10.1097/ALN.0000000000004777 PMID: 37934106
Alveolar Tipping Points in Changing Lungs Related to Positive End-expiratory Pressure
Tilo Winkler, Marcelo B P Amato.
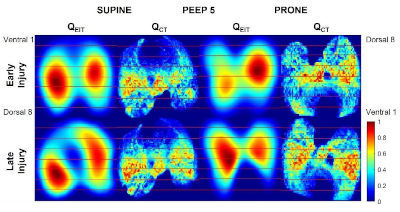
Research | Anesthesiology. (2023)139:815-826. doi: 10.1097/ALN.0000000000004731 PMID: 37566686
Electrical Impedance Tomography Identifies Evolution of Regional Perfusion in a Porcine Model of Acute Respiratory Distress Syndrome
Kevin T Martin, Yi Xin, Timothy G Gaulton, Marcus Victor, Roberta R Santiago, Taehwan Kim, Caio C A Morais, Aubrey A Kazimi, Marc Connell, Sarah E Gerard, Jacob Herrmann, Ariel L Mueller, Austin Lenart, Jiacheng Shen, Sherbano S Khan, Mihail Petrov, Kristan Reutlinger, Karina Rozenberg, Marcelo Amato, Lorenzo Berra, Maurizio Cereda.
CONCLUSIONS: Electrical impedance tomography closely approximated computed tomography perfusion measures in experimental acute respiratory distress syndrome, in the supine position, over injury progression and with increased PEEP. Further validation is needed to determine the accuracy of electrical impedance tomography in measuring perfusion redistributions after positional changes.
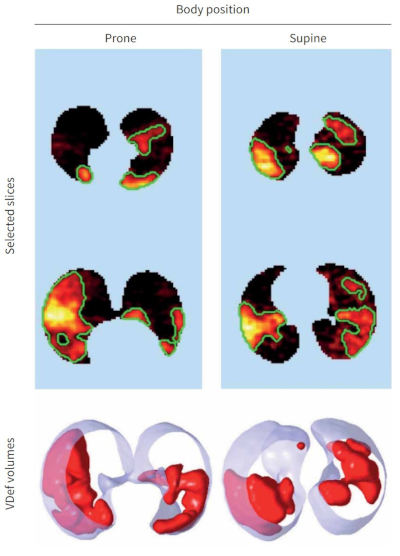
Breathe (Sheff). (2023)19:220272. doi: 10.1183/20734735.0272-2022 PMID: 38020338
Lung functional imaging
Sam Bayat, Jim Wild, Tilo Winkler.
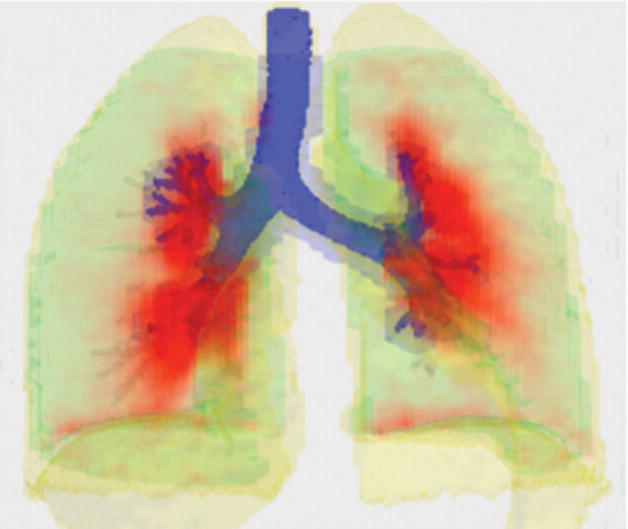
Review | J Aerosol Med Pulm Drug Deliv. (2023)36(4):210-227. doi: 10.1089/jamp.2023.29086.jgv PMID: 37585546
Measuring Anatomical Distributions of Ventilation and Aerosol Deposition with PET-CT
Jose G Venegas.
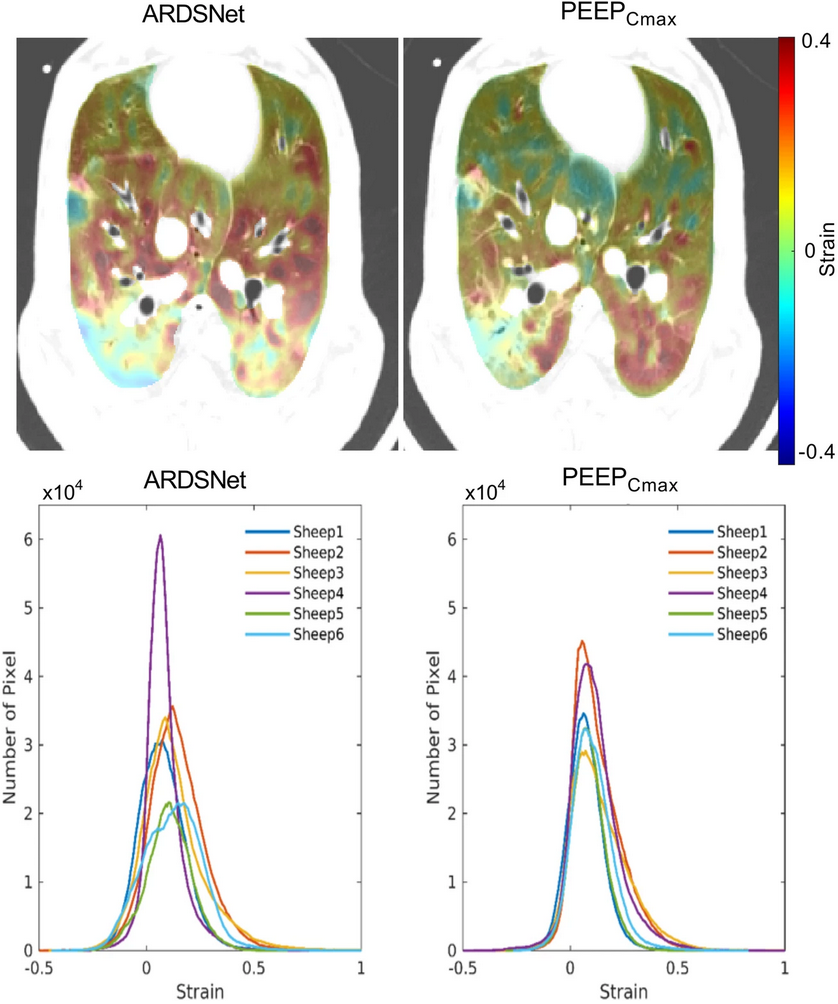
Research | Crit Care. (2023)27:307. doi: 10.1186/s13054-023-04591-7 PMID: 37537654
Dynamic lung aeration and strain with positive end-expiratory pressure individualized to maximal compliance versus ARDSNet low-stretch strategy: a study in a surfactant depletion model of lung injury
Congli Zeng, Min Zhu, Gabriel Motta-Ribeiro, David Lagier, Takuga Hinoshita, Mingyang Zang, Kira Grogg, Tilo Winkler, Marcos F Vidal Melo.
CONCLUSIONS: In well-recruitable ARDS models, a maximal compliance PEEP strategy improved end-expiratory/inspiratory whole-lung aeration and its homogeneity without overinflation. It further reduced dynamic strain in middle-ventral regions and tidal recruitment in middle-dorsal areas. These findings suggest the maximal compliance strategy minimizing whole-lung dynamically quantified mechanisms of ventilator-induced lung injury with less cyclic recruitment and no additional overinflation in large heterogeneously expanded and recruitable lungs.
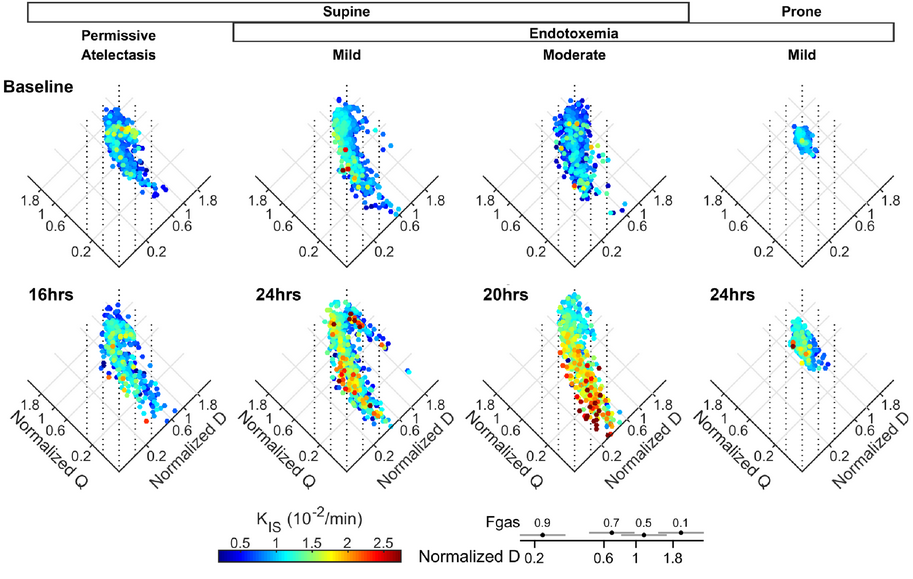
Research | J Appl Physiol. (2023)135:239-250; doi: 10.1152/japplphysiol.00028.2023 PMID: 37289955
Worsening of lung perfusion to tissue density distributions during early acute lung injury
Gabriel C Motta-Ribeiro, Tilo Winkler, Eduardo L V Costa, Nicolas de Prost, Mauro R Tucci, Marcos F Vidal Melo.
CONCLUSIONS: The distribution of perfusion to density is markedly worsened in the early 16–24 h of low-VT mechanical ventilation before established ARDS. Implementation of an oxygenation-based PEEP-setting ventilatory strategy resulted in worse lung expansion with susceptibility to mechanical injury when substantial reduction of perfusion to poorly aerated areas was present, i.e., in mild instead of moderate endotoxemia. Redistribution of perfusion-to-density was dependent on ventilatory strategy, level of endotoxemia and body position, and associated with regional neutrophilic inflammation. 13NN-saline PET assessments indicated that capillary collapse in the absence of right ventricular obliteration could contribute to such inflammation.
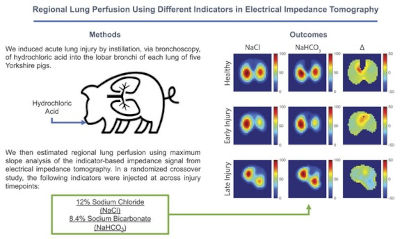
Research | J Appl Physiol. (2023)135:500-507. doi: 10.1152/japplphysiol.00130.2023 PMID: 37439236
Regional lung perfusion using different indicators in electrical impedance tomography
Timothy G Gaulton, Kevin Martin, Yi Xin, Marcus Victor, Roberta Ribeiro De Santis Santiago, Marcelo Britto Passos Amato, Lorenzo Berra, Maurizio Cereda.
CONCLUSIONS: The difference in regional lung perfusion between indicators did not change across experimental conditions. Sodium bicarbonate may be a comparable indicator to estimate regional lung perfusion using electrical impedance tomography.
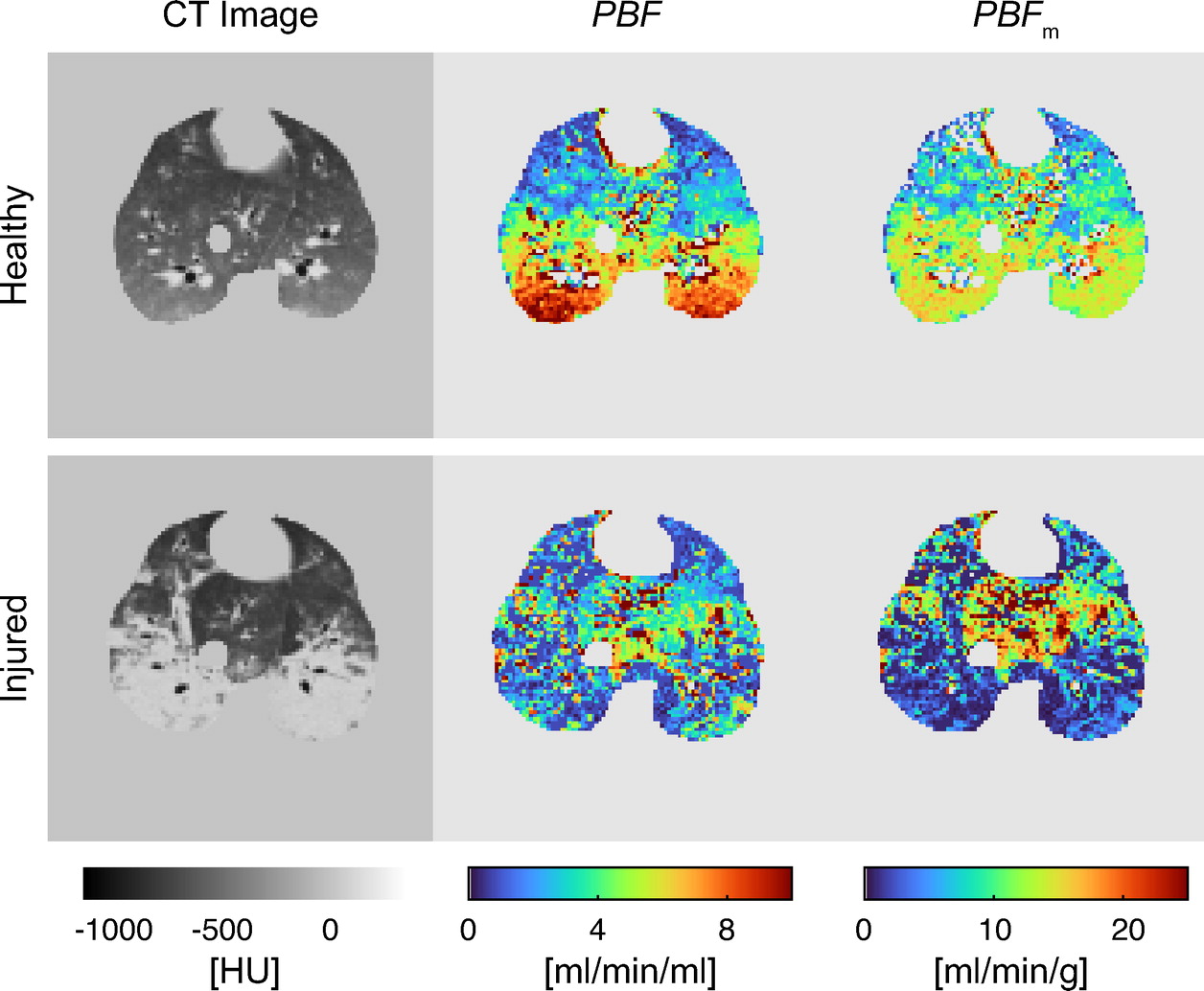
Research | J Appl Physiol. (2023)134:1496-1507; doi: 10.1152/japplphysiol.00159.2023 PMID: 37167261
Improving pulmonary perfusion assessment by dynamic contrast-enhanced computed tomography in an experimental lung injury model
Yi Xin, Taehwan Kim, Tilo Winkler, Gunnar Brix, Timothy Gaulton, Sarah E Gerard, Jacob Herrmann, Kevin T Martin, Marcus Victor, Kristan Reutlinger, Marcelo Amato, Lorenzo Berra, Mannudeep K Kalra, Maurizio Cereda.
CONCLUSIONS: Dynamic contrast-enhanced CT using a lower-viscosity contrast agent in combination with tracer-kinetic analysis by the steepest-slope model improves pulmonary blood flow measurements and assessment of regional distributions of lung perfusion. It is thus a promising technique that may be used in the future as a fast and reproducible imaging tool to characterize lung perfusion in ARDS.
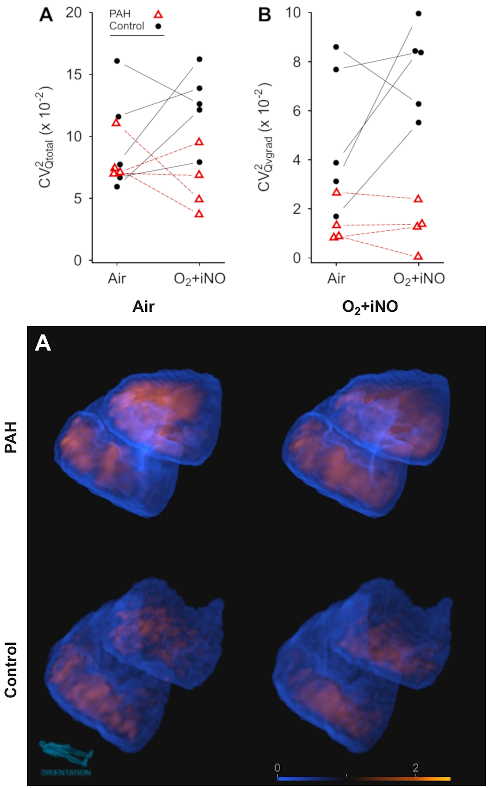
Research | Respiratory Research (2022)23:325; doi: 10.1186/s12931-022-02239-8 PMID: 36457013
Perfusion imaging heterogeneity during NO inhalation distinguishes pulmonary arterial hypertension (PAH) from healthy subjects and has potential as an imaging biomarker
Tilo Winkler, Puja Kohli, Vanessa J Kelly, Ekaterina G Kehl, Alison S Witkin, Josanna Rodriguez-Lopez, Kathryn A Hibbert, Mamary Kone, David M Systrom, Aaron B Waxman, Jose G Venegas, Richard Channick, R Scott Harris.
CONCLUSIONS: Perfusion imaging during O2 + iNO showed a significant difference in the heterogeneity associated with the vertical gradient in perfusion, distinguishing in this small cohort study PAH subjects from controls.

Editorial | J Allergy Clin Immunol. 2021 Jul 23;
Airway remodeling: shifting the trigger point for exacerbations in asthma
Tilo Winkler and Urs Frey. PMID: 34310927
Research | Am J Respir Cell Mol Biol. 2021 Jun 24. doi: 10.1165/rcmb.2021-0168OC. PMID: 34166603
Smoking and HIV-1 Infection Promote Retention of CD8+ T Cells in the Airway Mucosa
Björn Corleis, Josalyn L Cho, Samantha J Gates, Alice H Linder, Amy Dickey, Antonella C Lisanti-Park, Abigail E Schiff, Musie Ghebremichael, Puja Kohli, Tilo Winkler, R Scott Harris, Benjamin D Medoff, Douglas S Kwon.